Smart Pill Box
Learn More
Free Sample & Demo
Digital Adherence Technologies
Smart Pill Box
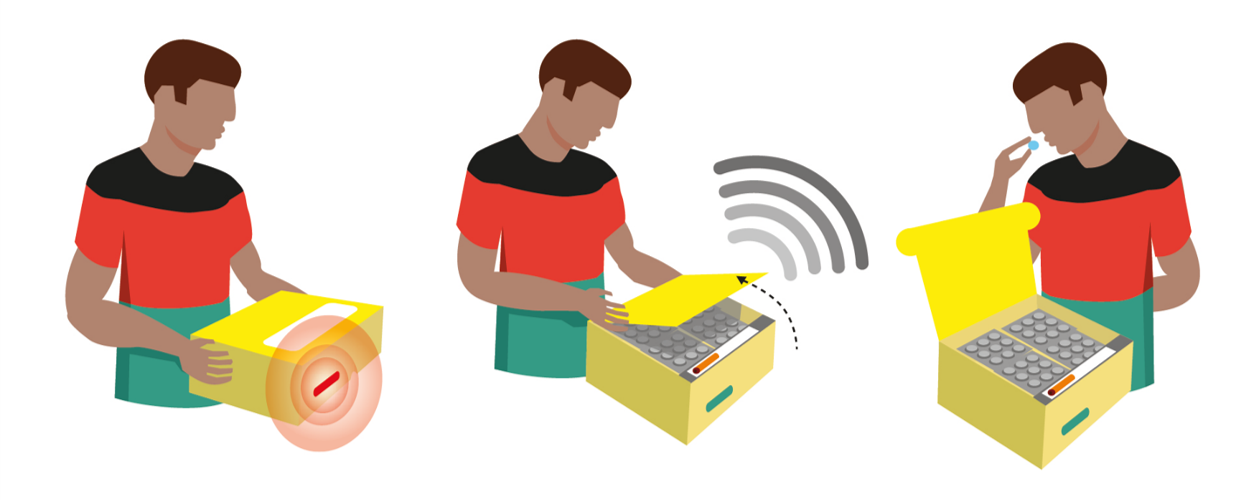
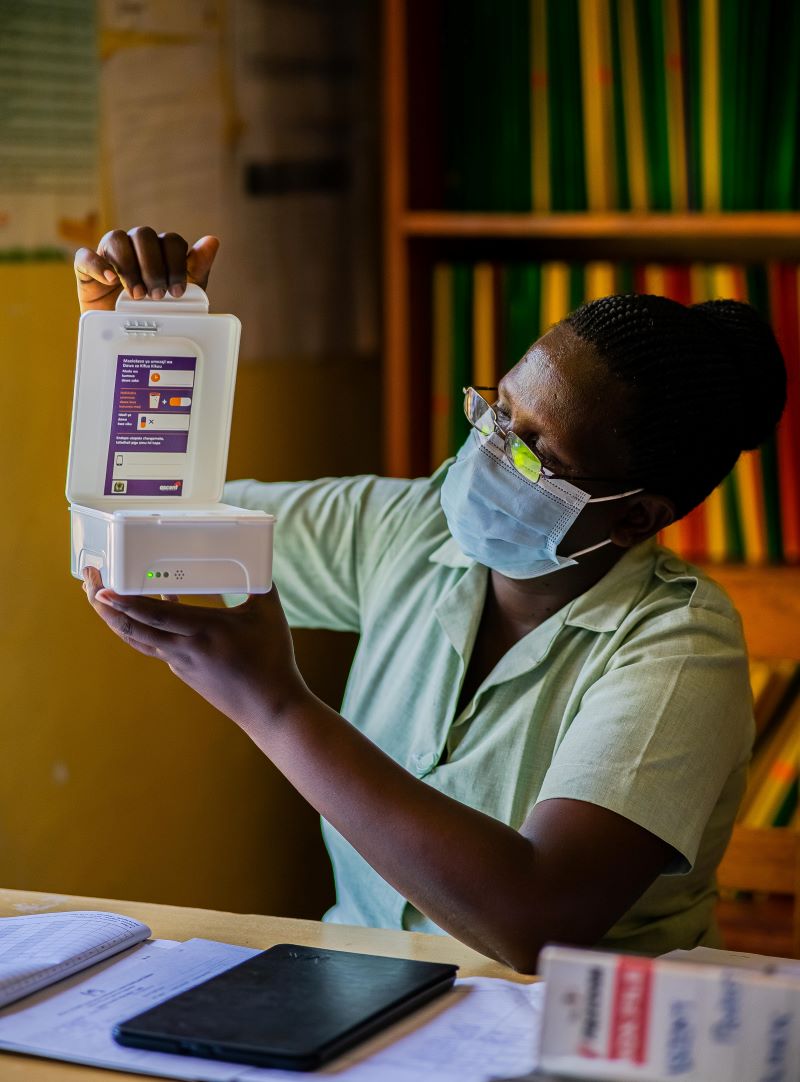
With the use of a low-cost medication container, a battery powered sensor and a mobile data connection, the smart pill box automatically logs patient medication intake each time the patient opens the box to take medication by sending a signal.
Features:
- Battery that lasts up to 6 months before it requires a recharge.
- Global mobile data subscription for 36 months.
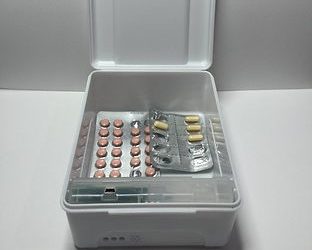
- Various container sizes available to store and organize up to a month’s supply of medication.
- LED and speaker to enable visual/audible dose reminders.
- Printed instructions can be added.
Considerations
To determine whether the smart pill box is suitable for target population and context, certain elements need to be considered.
Each consideration is scored according to a favourability scale as low, medium, or high. Low indicating less favourability, and high indicating increased favourability compared to the other digital adherence technologies.
These considerations are based on the ASCENT Project Technical Brief, summarizing the implementation experiences with three technologies in 5 countries.
EASE FOR PATIENT TO USE TECHNOLOGY: HIGH
- Medication intake is logged and sent automatically by opening the smart pill box.
- The size of the box reduces its portability and might increase the risk of stigmatization.
EASE FOR HEALTHCARE WORKER TO USE TECHNOLOGY: MEDIUM
- The smart pill box needs to be fully charged before it can be provided to patient
- The smart pill box can be re-used for multiple patients. This requires retrieval of the smart pill box from the patient, assessment of its functionality and cleaning of existing container or provisioning of new container.
ACCESSIBILITY: HIGH
- The patient doesn’t need any additional technology to be able to make use of this DAT – the box is provided by the healthcare facility.
- When mobile connectivity is temporary not available, opening events are stored in the smart pill box memory and uploaded automatically when internet connectivity is available again.
EASE OF IMPLEMENTATION: MEDIUM
- The smart pill box is available through the Global Drug Facility (GDF).
- Besides charging, the smart pill box can be used by patients immediately after delivery. No specialized in-country infrastructure required.
- Lead-time from ordering to receival of smart pill boxes in-country can take between 3 to 6 months.
- The smart pill box requires a 2G mobile network which might not be available in each country or region.
SUITABILITY FOR USE WITH DIFFERENT DRUG TYPES: HIGH
- Suitable across all medication types and/or combination of treatment(s).
Implementation Requirements
Follow-up based on adherence
Targeted follow-up actions and support by healthcare providers and other staff based on adherence.
Adherence platform access for provider
Healthcare provider can use smartphone, tablet, or laptop to access online adherence platform.
Costs
At $53.75 the kit includes:
- a plastic medication container
- batteries
- a chip and wireless data sufficient for at least three years — meaning it could be used by multiple patients over that period.
The plastic container can be cleaned or replaced for each patient, but the chip inside, which is the expensive component, can be transferred to a new container — saving government costs.
For more information use the following budget guidance: Budget Line Items for Inclusion in Funding Applications
Countries Implementing the Smart Pill Box
Botswana
Patient Target
MDR-TB patient contacts (prophylaxis)
Scale
300
Publications
Ethiopia
Patient Target
DS-TB
DR-TB
Scale
15090
DAT Combination
Smart pill box; Medication sleeve
Publications
Kenya
Patient Target
MDR-TB patient contacts (prophylaxis)
Scale
350
Publications
Mozambique
Patient Target
LTBI
Scale
Philippines
Patient Target
DS-TB
DR-TB
MDR-TB patient contacts (prophylaxis) (350 patients)
Scale
15350
DAT Combination
Smart pill box, medication sleeve, VOT
Publications
Thailand
Patient Target
MDR-TB patient contacts (prophylaxis) (350 patients)
Scale
160
Publications
Vietnam
Patient Target
DS-TB
Scale
44
Publications
Brazil
Patient Target
MDR-TB patient contacts (prophylaxis)
Scale
140
Publications
Haiti
Patient Target
MDR-TB patient contacts (prophylaxis)
Scale
100
Publications
Kyrgyzstan
Patient Target
DS-TB
MDR-TB
Scale
50
Nepal
Patient Target
DS-TB
Scale
200
Publications
South Africa
Patient Target
DS-TB
DR-TB
MDR-TB patient contacts (prophylaxis) (1590 patients)
Scale
22050
DAT Combination
Smart pill box, medication sleeve, VOT
Publications
Uganda
Patient Target
DS-TB
MDR-TB patient contacts (prophylaxis) (1590 patients) (260 patients)
Scale
337
Publications
Zimbabwe
Patient Target
MDR-TB patient contacts (prophylaxis) (1590 patients)
Scale
43
Publications
China
Patient Target
DS-TB
DR-TB
Scale
9227
DAT Combination
Smart pill box; VOT
Publications
India
Patient Target
DS-TB
DR-TB
MDR-TB
Scale
2218
Publications
Madagascar
Patient Target
DS-TB
Scale
445
Publications
Peru
Patient Target
MDR-TB patient contacts (prophylaxis)
Scale
687
Publications
Tanzania
Patient Target
DS-TB
DR-TB
Smear positive TB patients and presumptive TB cases (660 patients)
Scale
15660
Publications
Ukraine
Patient Target
DS-TB
DR-TB
Scale
19900
DAT Combination
Smart Pill Box, VOT
Publications
Begin Implementation
Publications
Technical Brief: 5 Country Experiences with Digital Adherence Technologies
Based on the experience of implementing digital adherence technologies at scale in five different contexts, this technical brief serves to
highlight and summarize the ASCENT project implementation experiences with 3 technologies.
Using electronic medication monitoring to guide differential management of tuberculosis patients at the community level in China
In settings such as China, where universal implementation of directly observed therapy (DOT) is not feasible, innovative approaches are needed to support patient adherence to TB treatment. The electronic medication monitor (EMM) is one of the digital technologies recommended by the World Health Organization (WHO), but evidence from implementation studies remains sparse. In this study, we evaluated acceptance of the EMM among health care workers and patients while implementing the device for differential TB patient management at the community level.
The impact of digital health technologies on tuberculosis treatment: a systematic review
Digital technologies are increasingly harnessed to support treatment of persons with tuberculosis (TB). Since in-person directly observed treatment (DOT) can be resource intensive and challenging to implement, these technologies may have the potential to improve adherence and clinical outcomes. We reviewed the effect of these technologies on TB treatment adherence and patient outcomes.
Pilot evaluation of a second-generation electronic pill box for adherence to Bedaquiline and antiretroviral therapy in drug-resistant TB/HIV co-infected patients in KwaZulu-Natal, South Africa
The introduction of Bedaquiline, the first new antimycobacterial drug in over 40 years, has highlighted the critical importance of medication adherence in drug-resistant tuberculosis (DR-TB) treatment to prevent amplified drug-resistance and derive sustained benefit. Real-time electronic dose monitoring (EDM) accurately measures adherence and allows for titration of adherence support for anti-retroviral therapy (ART). The goal of this study was to evaluate the accuracy and acceptability of a next-generation electronic pillbox (Wisepill RT2000) for Bedaquiline-containing TB regimens.
Usability of a Medication Event Reminder Monitor System (MERM) by Providers and Patients to Improve Adherence in the Management of Tuberculosis
Here we report a usability study of a newly-designed, modular electronic monitor product, called the MERM (Medication Event and Reminder Monitor), that is compatible with TB medication formats and supply chains in resource-limited settings. This study, done in a rural setting in China, showed that the use of the MERM for EM of TB medications was associated with a high degree of user performance, acceptability, and satisfaction among both TB patients and medical staff.
A randomised controlled trial to evaluate a medication monitoring system for TB treatment
BACKGROUND:
Adherence to TB treatment and therefore treatment success could be improved using digital adherence technology.
OBJECTIVE:
To evaluate the effectiveness of a medication event reminder monitor system (MERM) on treatment success and treatment adherence in patients with drug-susceptible pulmonary TB in Perú.